Evaluation process for active substances
Evaluation process for active substances
Once an application for approval of an active substance is considered to be valid by the evaluating competent authority, the evaluation process starts. The evaluating competent authority has 365 days to assess the application and provide its conclusion to ECHA.
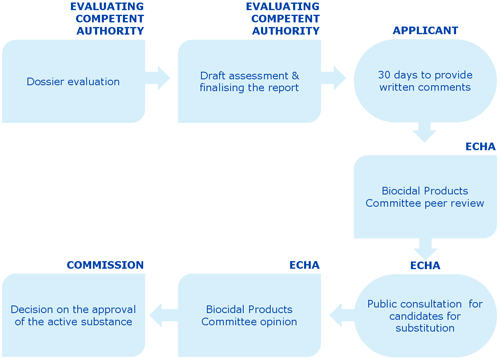
This graph shows an overview of the dossier evaluation process
During the evaluation process, the applicant may be requested to provide additional information if the evaluating competent authority considers that more information is necessary. The applicant has to provide the requested information within 180 days unless a delay is justified by the nature of the data requested or by exceptional circumstances.
The evaluation process has the following steps.

The evaluating competent authority carries out the dossier evaluation.
The evaluating competent authority finalises the draft assessment report and the conclusions of its evaluation.
The draft assessment report is sent to the applicant through R4BP 3. The applicant has 30 days to provide written comments.
The assessment report is transmitted through R4BP 3 to ECHA for peer review in the Biocidal Products Committee (BPC).

If the active substance is a candidate for substitution, a public consultation is launched. This gives third parties the possibility to submit relevant information, including information on alternative substances.
The BPC has 270 days to deliver an opinion through a peer review assessment and to submit this opinion to the Commission.

The Commission takes a decision on the approval of the active substance.
Following a positive decision, the active substance is included on the Union list of approved active substances.
The main actors in the evaluation process are:
Applicants
Applicants are responsible for providing all necessary information in their dossiers. They should pay attention to the various deadlines throughout the process. Applicants have the possibility to comment on the draft report of their dossier during the process.
Public
If the active substance is a candidate for substitution citizens, organisations, academics, companies or authorities have the possibility to submit relevant information during the public consultation procedure.
ECHA
ECHA coordinates the approval process and provides the necessary tools and support for the applicants. ECHA also provides the Secretariat for the Biocidal Products Committee.
Biocidal Products Committee (BPC)
The Biocidal Products Committee gives a scientific opinion on active substances (approval, renewal, review, inclusion in Annex I), Union authorisation of biocidal products and mutual recognition. The Committee also deals with scientific and technical matters and other questions at the request of the European Commission and the Member States. The Committee consists of members appointed by EU Member States and EEA countries on the basis of their experience.
Evaluating competent authorities
The evaluating competent authority is responsible for carrying out the evaluation of the applications. The evaluating competent authority is chosen by the applicant.
European Commission
The European Commission together with the Standing Committee on biocidal products, takes into consideration the opinion issued by the BPC and decides whether to approve the active substance or not. The Standing Committee is chaired by the Commission and has representatives from all Member States. Following a positive decision, the Commission includes the active substance on the Union list of approved active substances..